Our customers work more efficiently and benefit from
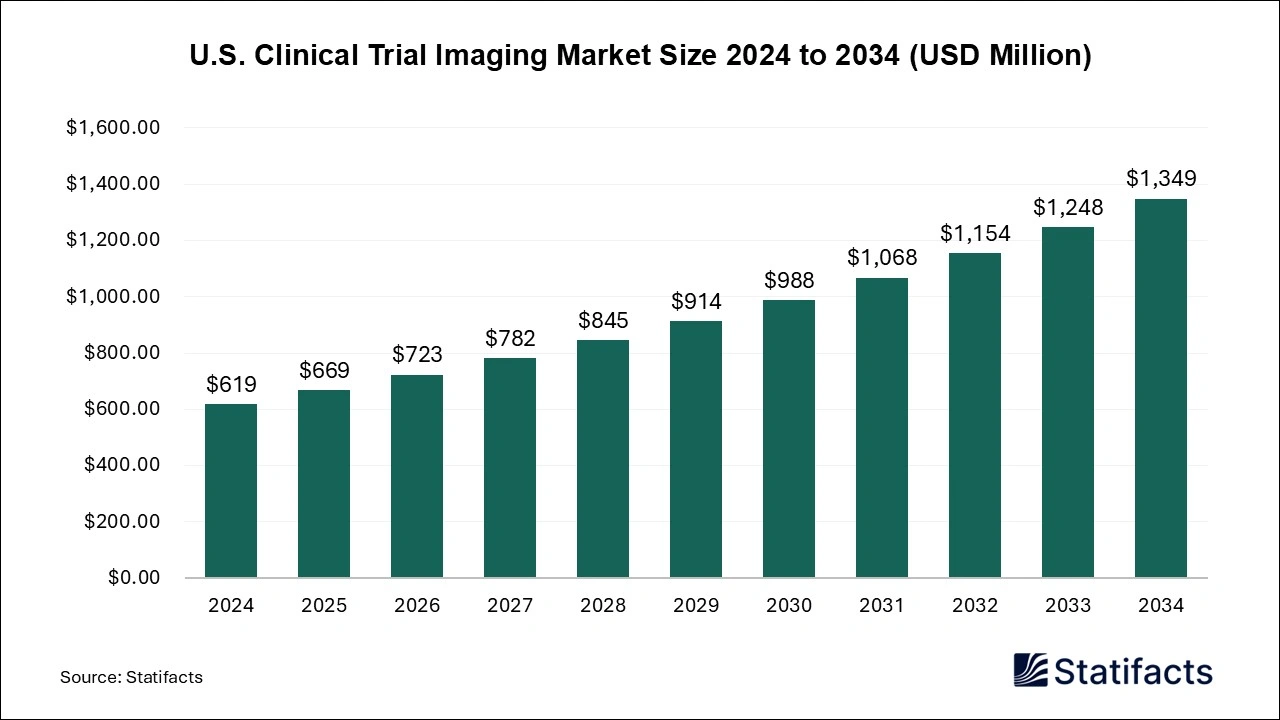
The U.S. clinical trial imaging market size was exhibited at USD 619 million in 2024 and is projected to hit around USD 1,349 million by 2034, growing at a CAGR of 8.1% during the forecast period 2024 to 2034.
The U.S. clinical trial imaging market is thriving owing to the dominance of the country in conducting clinical trials within the state as well as on a global scale. The presence of advanced healthcare infrastructure, cutting-edge medical imaging technologies, huge investments in development of innovative imaging techniques integrated with artificial intelligence (AI) tools for improving efficiency and reliability for clinicians are the factors driving the market growth. Additionally, almost 50% of clinical trials require imaging technologies which ultimately contributes in accelerating the drug development process for clinical researchers.
Clinical trial imaging refers to the utilising imaging applications for visual assessment of disease progression, monitoring efficacy of treatment, identification of potential patient responders and also in early disease detection. Various imaging techniques are used in clinical trials including X-ray based imaging, cross-sectional imaging techniques (MRI, CT), ultrasound techniques, ophthalmic imaging and camera-based tools for screening and diagnosis of disease conditions.
Screening imaging clinical trials is crucial for researchers to ensure the best imaging technique for early detection of disease, likely even before the symptoms of the disease kick in. Moreover, diagnostic imaging clinical trials help in motoring the disease and checking the treatment efficacy. Whereas, image-guided interventional clinical trials evaluate the function of therapies directed by imaging techniques.
The clinical trial imaging techniques greatly support the drug development process in clinical trials as they are widely accepted in participants due to their non-invasive nature and also act as surrogate biomarkers for evaluating disease progression or response which is approved by the regulatory agencies. Furthermore, they are cost-effective in long term studies and significantly reduce the drug development and approval period.
However, the concerns regarding safety of patient data, standardization of imaging for consistent results, unified review across multiple sites and insufficiency in resource investment is creating challenges for the U.S. clinical trial imaging market. Furthermore, restricted accessibility to sites with latest imaging technologies, need for fast and reliable sharing of data among CROs and sponsors and the harmonization of imaging biomarkers across sites is restraining the market growth.
The challenges linked to imaging in clinical trials can be addressed by contacting professionals at specialized CROs for managing and completing the imaging process while adhering to the standards and regulatory requirements thereby streamlining the image acquisition, analysis, interpretation and transfer process.
Furthermore, the growing support from the U.S. government for clinical trial imaging by implementing several initiatives such as Medical Imaging and Diagnostics Program by the FDA’s Center for Devices and Radiological Health (CDRH), Critical Path Initiative, FDA approvals for clinical trials research projects, funding’s from the National Institute of Health (NIH) and the initiative for reducing unnecessary exposure to radiation through medical imaging.
The integration of AI and machine learning tools in clinical trials imaging is extensively being applied across the U.S. for automating image analysis and quality control, for lesion segmentation and measurement, screening of eligible patients, prognostic modelling and for enhancing the accuracy in identifying disease biomarkers which will essentially accelerate the clinical trial process while refining data quality. Nevertheless, issues regarding FDA approvals for AI-driven medical imaging tools and strict regulations imposed by Health Insurance Portability and Accountability Act (HIPAA) for the handling of patient data used in AI development and analysis should be considered.
Following standardized protocols across all study sites for ensuring data equivalence and consistency when analysing image parameters, implementation of blinding procedures for minimizing bias in interpretation and utilizing advanced analysis software for assessing imaging features and extraction of applicable data will significantly boost the reliability on imaging techniques for clinicians as well as patients.
Rising demand for precision diagnostics, increased shift towards decentralized clinical trials, technological advancements in diagnostic imaging procedures, influence of constant regulatory changes, growing use of imaging techniques in oncology clinical trials for decision making with evidence and surge in registration cohorts is driving the growth opportunities for this market.
For any questions about this dataset or to discuss customization options, please write to us at sales@statifacts.com
| Stats ID: | 7623 |
| Format: | Databook |
| Published: | January 2025 |
| Price | US$ 1550 |
Immediate Delivery

| Stats ID: | 7623 |
| Format: | Databook |
| Published: | January 2025 |
| Price | US$ 1550 |
Immediate Delivery

You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.

Unlock unlimited access to all exclusive market research reports, empowering your business.
Get industry insights at the most affordable plan
Stay ahead of the competition with comprehensive, actionable intelligence at your fingertips!
Learn More